The Critical Role of Activated Alumina in Fluoride Removal Projects: Principles, Efficiency, and Case Analysis
Meta Description: Discover how Activated Alumina works as a superior fluoride adsorbent. This technical analysis covers its removal mechanism, key efficiency factors, and a real-world case study on achieving safe drinking water standards.
Introduction
Access to safe drinking water is a global priority, and elevated fluoride levels pose a significant health risk to millions worldwide. Among the various defluoridation technologies, Activated Alumina (AA) has emerged as a proven, efficient, and reliable adsorption medium. This article delves into the critical role of Activated Alumina in fluoride removal projects, exploring the fundamental principles behind its efficacy, the factors influencing its performance, and a practical case study demonstrating its successful application.
1. The Working Principle: How Activated Alumina Adsorbs Fluoride
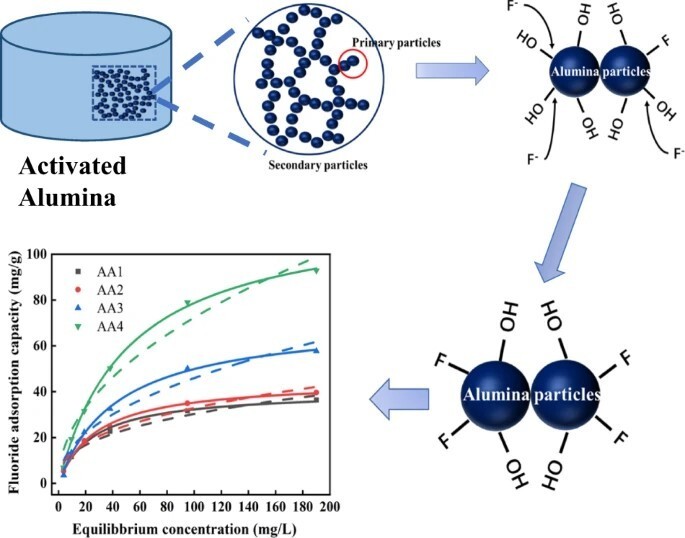
Activated Alumina is not a simple filter; it is a highly porous form of aluminum oxide (Al₂O₃) with an immense surface area. Its defluoridation capability is rooted in ion exchange and surface adsorption.
Surface Properties: The surface of Activated Alumina is covered with hydroxyl groups (-OH). In an aqueous environment, these groups become active sites for ion exchange.
The Ion Exchange Process: When fluoride-rich water passes through a bed of Activated Alumina, fluoride ions (F⁻) in the water swap places with the hydroxyl ions (OH⁻) on the AA surface. This process effectively traps the fluoride ions onto the adsorbent.
High Selectivity: The structure and electrochemistry of Activated Alumina give it a high affinity for anions like fluoride, arsenic, and selenium, making it exceptionally selective even in the presence of other ions.

2. Factors Influencing Removal Efficiency and Operational Design
The performance of an Activated Alumina filtration system is not absolute; it is optimized by controlling several key parameters:
pH Level: The adsorption is most effective in a slightly acidic pH range (typically 5.0 to 6.0). Outside this range, efficiency drops significantly.
Empty Bed Contact Time (EBCT): This is the critical contact time between water and the adsorbent. A longer EBCT (usually 5-10 minutes) ensures thorough contact and higher fluoride removal.
Initial Fluoride Concentration: Higher initial concentrations will exhaust the adsorbent more quickly, affecting the bed life and regeneration cycle.
Competing Ions: The presence of other anions, such as sulfates and carbonates, can compete for adsorption sites, potentially reducing the system's capacity for fluoride.
3. A Practical Case Analysis: Community Water Supply Project in [Example: Southeast Asia]
Background: A rural community in [Example Country] faced a severe health crisis due to groundwater fluoride concentrations of 6.5 mg/L, far exceeding the WHO guideline of 1.5 mg/L.
Solution Implemented:
A centralized water treatment plant was commissioned, featuring two large-scale Activated Alumina adsorption towers operating in series.
Process & Results:
Pre-treatment: Incoming water was first acidified to adjust the pH to an optimal 5.5.
Adsorption: Water was then pumped through the towers with a designed EBCT of 8 minutes.
Post-treatment: The treated water was neutralized before being supplied to the distribution network.
Outcome: The system consistently produced effluent with fluoride levels below 1.0 mg/L. The fluoride removal efficiency was calculated to be over 92%.
Regeneration: Once exhausted, the Activated Alumina was regenerated using a dilute caustic soda (NaOH) solution, followed by an acid rinse, restoring over 85% of its original capacity for multiple cycles, making the project highly cost-effective.
Why Activated Alumina Was Chosen Over Alternatives?
The project engineers selected Activated Alumina over reverse osmosis or other adsorbents due to its:
Proven track record for community-scale projects.
Lower operational cost compared to membrane technologies.
Robustness and ability to be regenerated multiple times.
Conclusion
Activated Alumina remains a cornerstone technology in the fight against fluoride contamination. Its well-understood mechanism, tunable efficiency, and demonstrable success in real-world projects make it an indispensable choice for engineers and policymakers. For municipalities and industries seeking a reliable, efficient, and economically viable solution for fluoride removal, Activated Alumina provides a critical line of defense in ensuring water safety.
Keywords & Phrases:
Activated Alumina, Fluoride Removal, Defluoridation, Water Treatment, Adsorbent, Ion Exchange, Drinking Water Safety, Case Study, Activated Alumina Regeneration, Groundwater Treatment, Fluoride Adsorption, Water Purification Technology.
Can't find what you're looking for ?
Leave a Message we will call you back quickly!
Get in Touch
*We respect your confidentiality and all information are protected.